MARKET ACCESS SUPPORT IN CEE
Differences in health systems among Central and Eastern European (CEE) countries reveal both the opportunities and uncertainties faced during the process of accessing the market with a new drug. The CEE region is characterized by its wide range of approaches to granting public funding for drugs – from a “no submission and no HTA” approach up to an extensive HTA with a systematic review and economic evaluation. The aim was to create and test the methodological approach to the assessment of P&R potential in CEE countries. The further goal was to identify countries with the highest reimbursement potential for a particular drug in the region and give our possibility to receive a full Market Access Support in CEE.
EU and CEE leader in Market Access and Real World Evidence Solutions
MARKET ACCESS SUPPORT
for CEE countries
CEE countries vary widely in their approaches for granting public funding for drugs from a ‘no submission and no HTA’ approach, to an extensive HTA with a systematic review and economic evaluation. To better understand the opportunities existing in the region for a given drug, we developed a methodology to assess Market Access with P&R potential in the CEE region.
Integrated Market Access Support in CEE region, with pathways, timelines recognition (action points) and assessment of probability of success in each pathway:
- Public funding decision making process for technologies
- Stakeholders mapping – most important stakeholders and their position in the system:
- Identifying Key Opinion Leaders – medical centers of importance
- Identifying Key Influencers reimbursement decisions in its country
- Possibilities of obtaining financing – priorities of the MOH, maps of health needs, current Health Policy Programs (including drug programs, Early Access Programs, Named Patient Access, targeted import)
- Analysis of available public funding schemes and conditions
- Mandatory P&R requirements in regard to submission and HTA dossier
- Pathways and timelines for reimbursement (action points)
- Market landscape analysis, with particular regard to comparators, including:
- Reimbursement status
- Method of financing
- Time to reimbursement
- Pricing
- Access restrictions
- Unmet need and burden of disease
- Clinical guidelines
- Recommendation on the optimum reimbursement submission mode
MARKET ACCESS DATA FROM FIVE DOMAINS

Estimation of the number of patients based on:
- Size of the population
- General prevalence
- Patients currently under care in a given system
Possibility to achieve average European list price:
- Adjustment: public funding and price for other therapies
- % relation to minimal official price in public funding calculate for each medical technology
- For each country, average of % relations will be calculate
Estimation of probability of public funding:
- 5-point categorization scale use: very low / low / moderate / high / very high
- Based on the general estimates and the number of reimbursed drugs/medical devices
Estimation of time to achieve public funding:
- Below 0.5 year / 0.5-1 year / 1-2 years / above 2 years
- Based on historical data on previous reimbursement AEDs
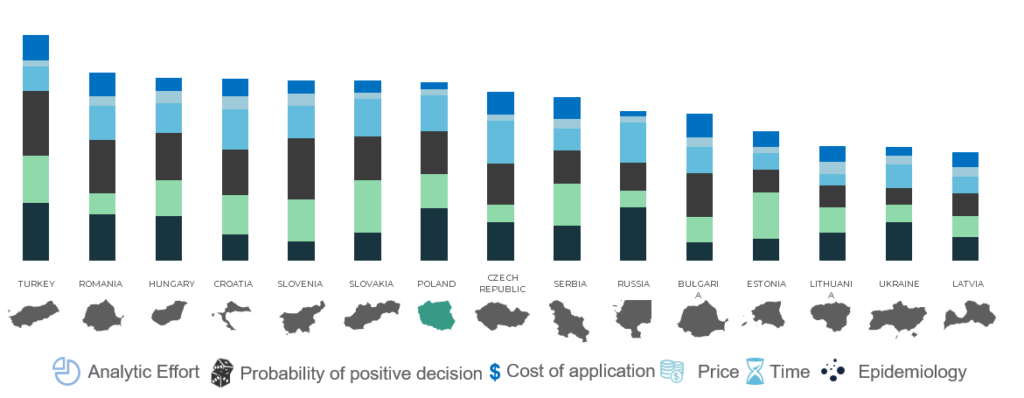
Additional categories:
- Analytic effort – level of extensiveness and complication of the analytic dossier
- Cost of application – including administrative cost and cost of the dossier
Multiple solutions for Market Access Support in CEE region
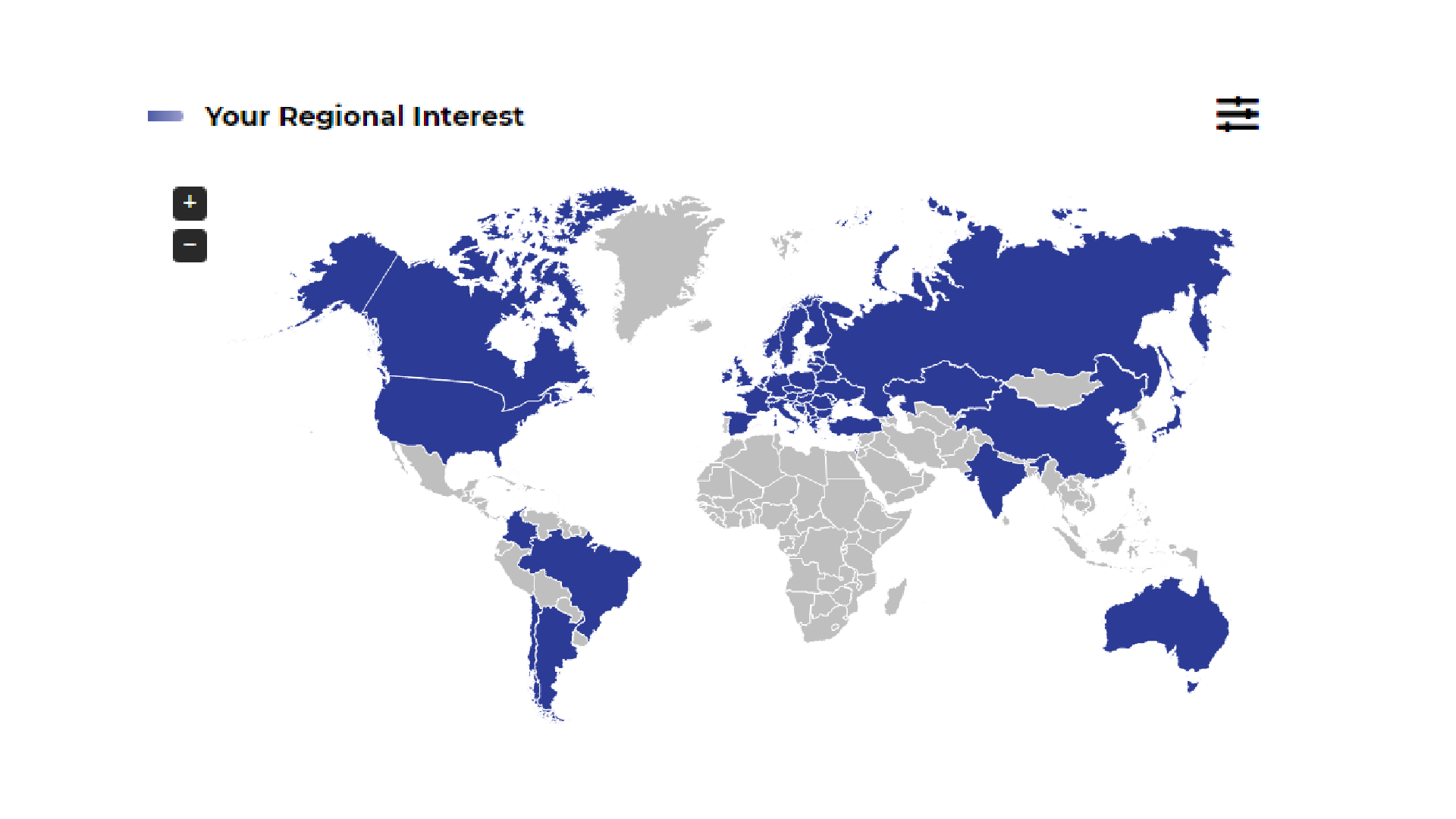
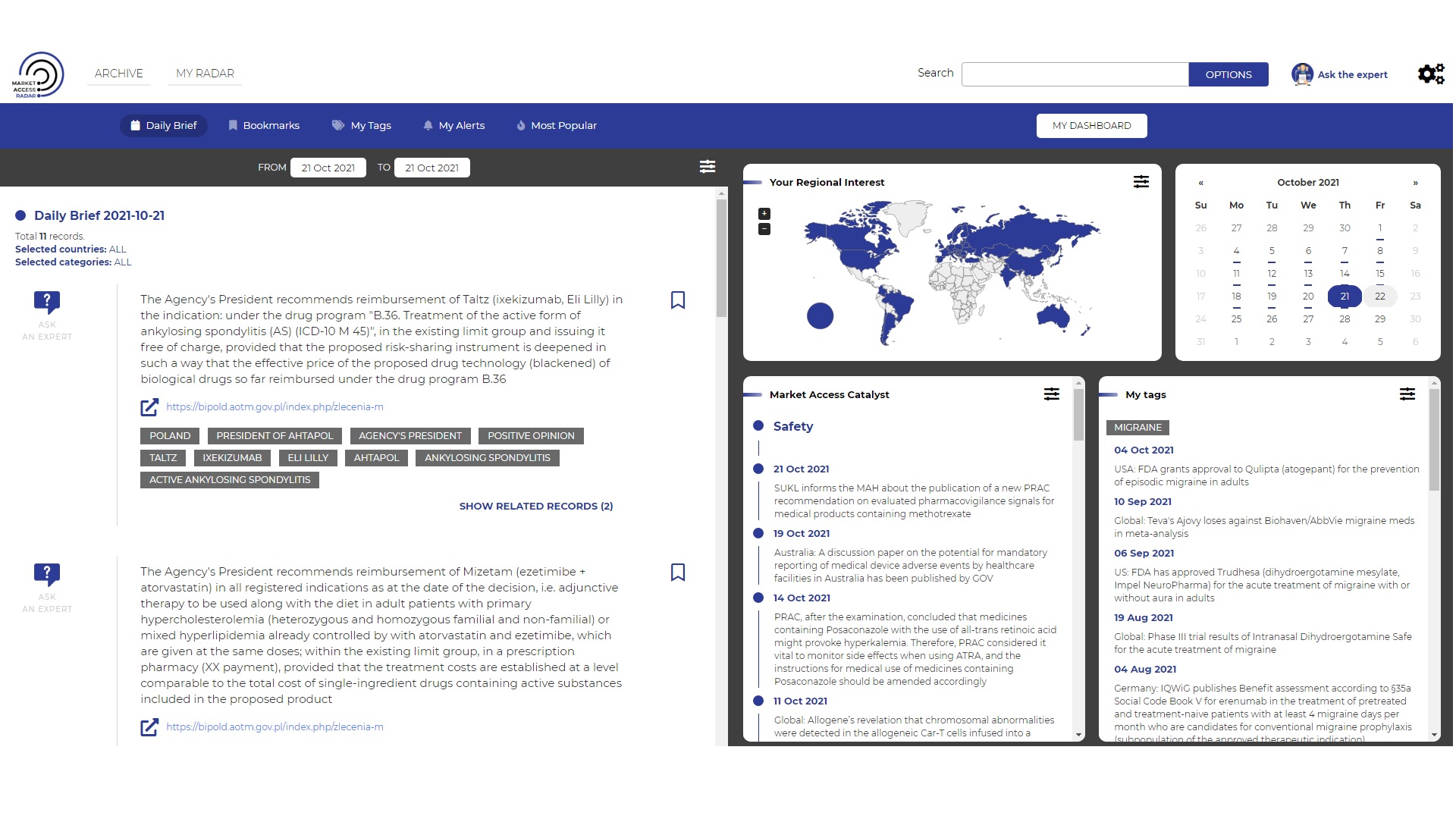
Market Access Radar (MAR) Repository
was created to provide you with quick and easy access to the most important MA-related news (from EU and CEE region). The portal is dedicated for the professionals from medical and pharmaceutical industry involved in widely understood Market Access.
MAR is based on constant monitoring, verification and a careful selection of >600 sources of information and data:
- The latest reimbursement recommendations, practices and pathways
- News on most recent study results, new marketing authorizations, drug safety, variations in registered and reimbursed indications published by EMA, NICE, FDA, pCORD, HAS, PBAC, AHTAPoL, Ministries of Health and other national institutions from CEE
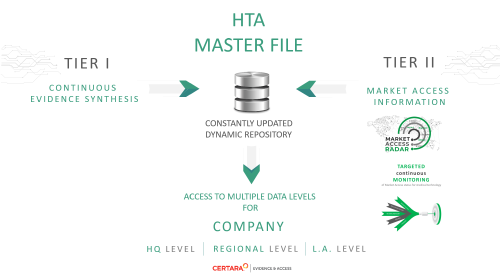
The HTA Master File
follows the EUnetHTA HTA dossier template (due to its wide recognition) and is a kind of HTA core model dedicated for defined products.
The primary purpose of the HTA Master File is to gather all of the information that is necessary to compose an HTA report into one comprehensive document, forming the basis for creating a dossier that responds to local market access needs (e.g. for the countries of Central and Eastern Europe).
Find it appealing?
For more information about our services, visit our website or contact us directly:

